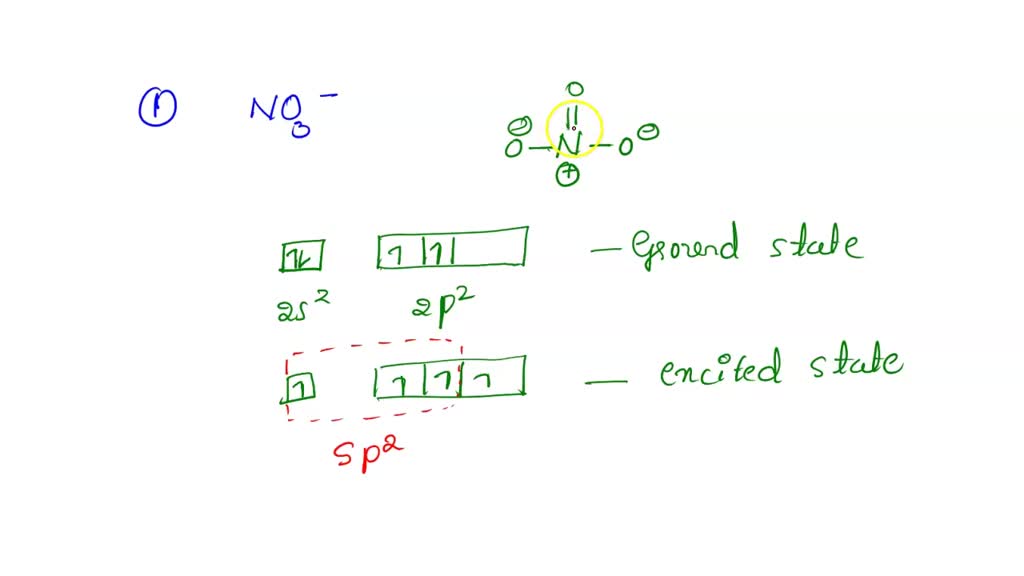
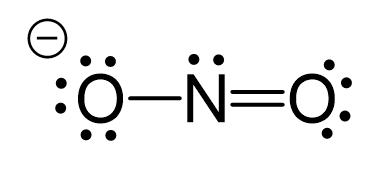The hybridization of atomic orbitals of nitrogen in NO2^+ , NO^-2 and NH4^+ are - Sarthaks eConnect | Largest Online Education Community
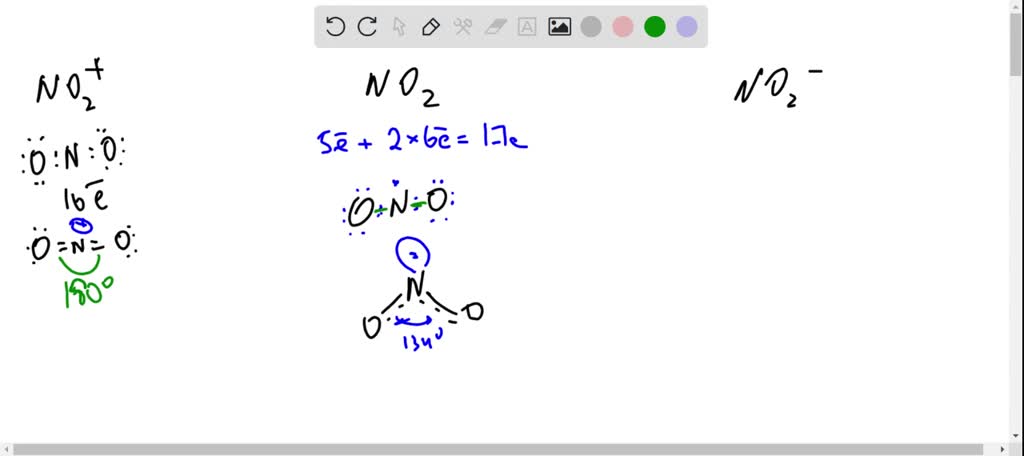
SOLVED: The three species NO2+, NO2 and NO2- all have a central N atom. The O-N-O bond angles in the three species are 180, 134 and 115 respectively. Explain this variation in

The hybridization of atomic orbital of nitrogen in NO2+, NO3- and NH4+ are:a)sp, sp2, sp3b)sp2, sp3, spc)sp2, sp, sp3d)sp, sp3, sp2Correct answer is option 'A'. Can you explain this answer? - EduRev Chemistry Question
![The types of hybrid orbitals of nitrogen in \\[NO_{2}^{+}\\], \\[NO_{3}^{-}\\] and \\[NH_{4}^{+}\\] respectively are expected to be:A. \\[sp,\\text{ }s{{p}^{3}}\\text{ }and\\text{ }s{{p}^{2}}\\]B. \\[sp,\\text{ }s{{p}^{2}}\\text{ }and\\text{ }s{{p}^{3 ... The types of hybrid orbitals of nitrogen in \\[NO_{2}^{+}\\], \\[NO_{3}^{-}\\] and \\[NH_{4}^{+}\\] respectively are expected to be:A. \\[sp,\\text{ }s{{p}^{3}}\\text{ }and\\text{ }s{{p}^{2}}\\]B. \\[sp,\\text{ }s{{p}^{2}}\\text{ }and\\text{ }s{{p}^{3 ...](https://www.vedantu.com/question-sets/34556bfa-0533-4f6a-a822-b7426c5f37ea4031147211903642416.png)
The types of hybrid orbitals of nitrogen in \\[NO_{2}^{+}\\], \\[NO_{3}^{-}\\] and \\[NH_{4}^{+}\\] respectively are expected to be:A. \\[sp,\\text{ }s{{p}^{3}}\\text{ }and\\text{ }s{{p}^{2}}\\]B. \\[sp,\\text{ }s{{p}^{2}}\\text{ }and\\text{ }s{{p}^{3 ...

In which of the following pairs of molecules/ions, the central atoms have sp^{2} hybridization?BF_{3} and NO{_{2}}^{-}NO{_{2}}^{-} and NH_{3}BF_{3} and NH{_{2}}^{-}NH{_{2}}^{-} and H_{2}O

NO2- , NO2, NO2+ Lewis dot structure, Identification of Co-ordinate Bond and hybridisation - YouTube